Do I need a skin biopsy or DaTscan for my Parkinson's disease?
By Michael S Okun MD
A lot of buzz has been generated as a result of the emergence of skin biopsies and DaTscans as potential diagnostic tests for Parkinson’s disease and for ‘parkinsonisms.’ The promise and the hope of these tests has been accompanied by ‘confusion’ as to ‘what they are’ and ‘when to apply them.’ In this month’s parkinsonsecrets.com blog, I will review the key information persons with Parkinson’s disease, family members and clinicians should all ‘know’ about these two diagnostic tests. Additionally, I will touch on the roles that these ‘tests’ may play in the future development of new therapies for Parkinson’s disease.
SPOILER ALERT: If you already have a diagnosis of levodopa responsive Parkinson’s disease, you may not require a skin biopsy or a DaTscan!
This picture shows how skin biopsies can be performed for various neurological and other diseases. The picture is from a nice 2019 article by Benson which appeared in Biomedical Microdevices. The second illustration is a representative picture of a DaTScan which is from another nice article published by Gallagher in Practical Neurology in 2019.
What is this new ‘skin biopsy’ for Parkinson’s disease?
It turns out that Parkinson’s disease is not limited to the brain. Evidence has been uncovered that Parkinson’s disease affects many of our body’s organ systems. The largest organ in the body is the ‘skin.’ Believe it or not, it turns out there are ‘depositions’ of Parkinson’s related proteins in the skin.
Thus, researchers have been interested in using skin biopsies as a method to identify and to quantify the alpha-synuclein contained in the skin. Multiple research groups around the world have been interested in obtaining skin samples and in using them to identify the ‘abnormal Parkinson’s protein;’ referred to as alpha-synclein. Researchers are seeking to use this skin ‘marker’ for diagnosis and possibly to monitor disease progression. Some experts refer to this as a ‘biomarker.’
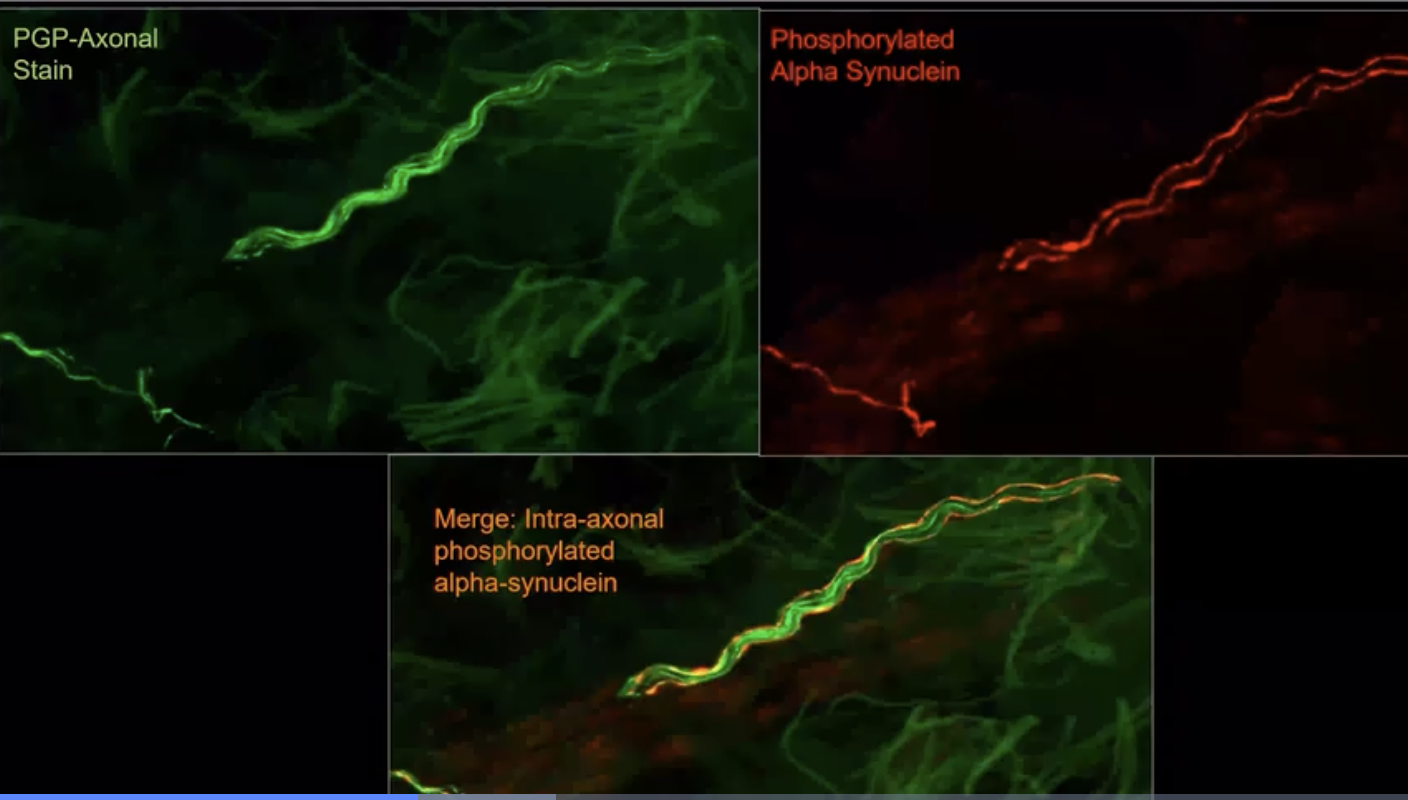
Above is an example of what staining of the skin biopsies looks like under the microscope from the Gibbons et al group who have been ‘early pioneers’ in this technology and in this skin based approach.
The Syn-One Test was developed by a company called CND Life Sciences to detect the Parkinson’s related protein alpha-synuclein (from multiple skin biopsies). The test focuses on detection of the ‘phosphorylated form of alpha-synuclein.’ This test is most accurate when ‘three’ tiny skin biopsies are obtained; each approximately the size of ~1/4 of a pencil eraser. These are collected and mailed away for a ‘pathological diagnosis.’ The biopsies are sampled from a person’s neck, thigh and ankle regions. The procedure takes ~10 minutes to perform, and the clinician frequently uses numbing medicine to make the experience as ‘painless as possible.’
The Syn-One Test is the first commercially available skin-related Parkinson’s related test. The test which can be performed by your doctor or clinician is classified as a “Laboratory Developed Test” (LDT), and thus is NOT FDA approved. The company providing the test (CND Life Sciences) has a laboratory regulated by CLIA and accredited by the College of American Pathologists. The idea of a LDT in clinical practice is actually common. LDT CLIA regulated tests can be used to detect proteins or other compounds and they operate without the requirement of FDA approval.
Researchers have observed that the location, the thickness and the laboratory methods applied may impact the results of a skin test. Also in fairness, many research groups worldwide have been and continue to develop skin, parotid and other biopsy technologies for Parkinson’s disease. I am focusing on Syn-One for my blog, because it is ‘currently’ the only one with CLIA approval (not FDA approval).
For those of you ‘geeking out’ and wanting to know more about what CLIA is and what it stands for check out the CMS website: The Centers for Medicare & Medicaid Services (CMS) “regulates all laboratory testing (except research) performed on humans in the U.S. through the Clinical Laboratory Improvement Amendments (CLIA). In total, CLIA covers approximately 320,000 laboratory entities. The Division of Clinical Laboratory Improvement & Quality, within the Quality, Safety & Oversight Group, under the Center for Clinical Standards and Quality (CCSQ) has the responsibility for implementing the CLIA Program. The objective of the CLIA program is to ensure quality laboratory testing. Although all clinical laboratories must be properly certified to receive Medicare or Medicaid payments, CLIA has no direct Medicare or Medicaid program responsibilities.”
This picture is from Chris Gibbon’s NIH work shows how they obtain 3 small biopsies to use for the Parkinson’s skin test. Here, Gibbons shows the neck, thigh and ankle locations. He presented this work at the AAN 2023 in Boston.
The Syn-One test is a CLIA approved ‘tissue based test’ to determine whether a sample contains alpha-synuclein. The test is not labelled for the diagnosis of Parkinson’s disease and is not FDA approved. The test can be ordered and billed to Medicare and to many insurance carriers; however the test’s indications must be documented by the clinician performing the biopsy.
Can the skin test differentiate between different forms of parkinsonism?
The many different forms of parkinsonism were nicely summarized in this table by Broski which appeared in a Radiographics article in 2014.
The Syn-One test has not been shown to reliably differentiate between parkinsonian syndromes and thus cannot by itself be used to diagnose and to differentiate Parkinson’s disease. The presence of phosphorylated alpha-synuclein in a skin biopsy is highly suggestive of the presence of a parkinsonian syndrome. There is ongoing research on how this ‘skin based’ data could be used alone or in combination with other tests to diagnose specific forms of parkinsonism.
What were the ‘preliminary’ results from the NIH study of the Syn-One skin test?
The preliminary results of the Syn-One study were presented at the 2023 AAN annual meeting in Boston by Dr. Christopher Gibbons.
The preliminary results for the NIH Syn-One test were presented at the 2023 American Academy of Neurology meeting in Boston by Dr. Christopher Gibbons, who is a professor of neurology at Harvard Beth Israel Deaconess Medical Center. Dr. Gibbon’s team used diagnostic criteria and classified a large group of persons with either Parkinson’s disease, MSA, Dementia with Lewy Bodies or with Primary Autonomic Failure (PAF).
The Syn-One test was applied to this group and was shown to have high sensitivity and specificity for picking up phosphorylated synculein in each of these four disease states. The preliminary results do not directly address whether the test will be able to differentiate between each of the four syndromes. We look forward to the full publication and to more information on this important point.
Finally, the results revealed a very low rate of adverse events from performing a skin biopsy in the setting of Parkinson’s disease.
What else could a skin biopsy tell us besides presence of synuclein?
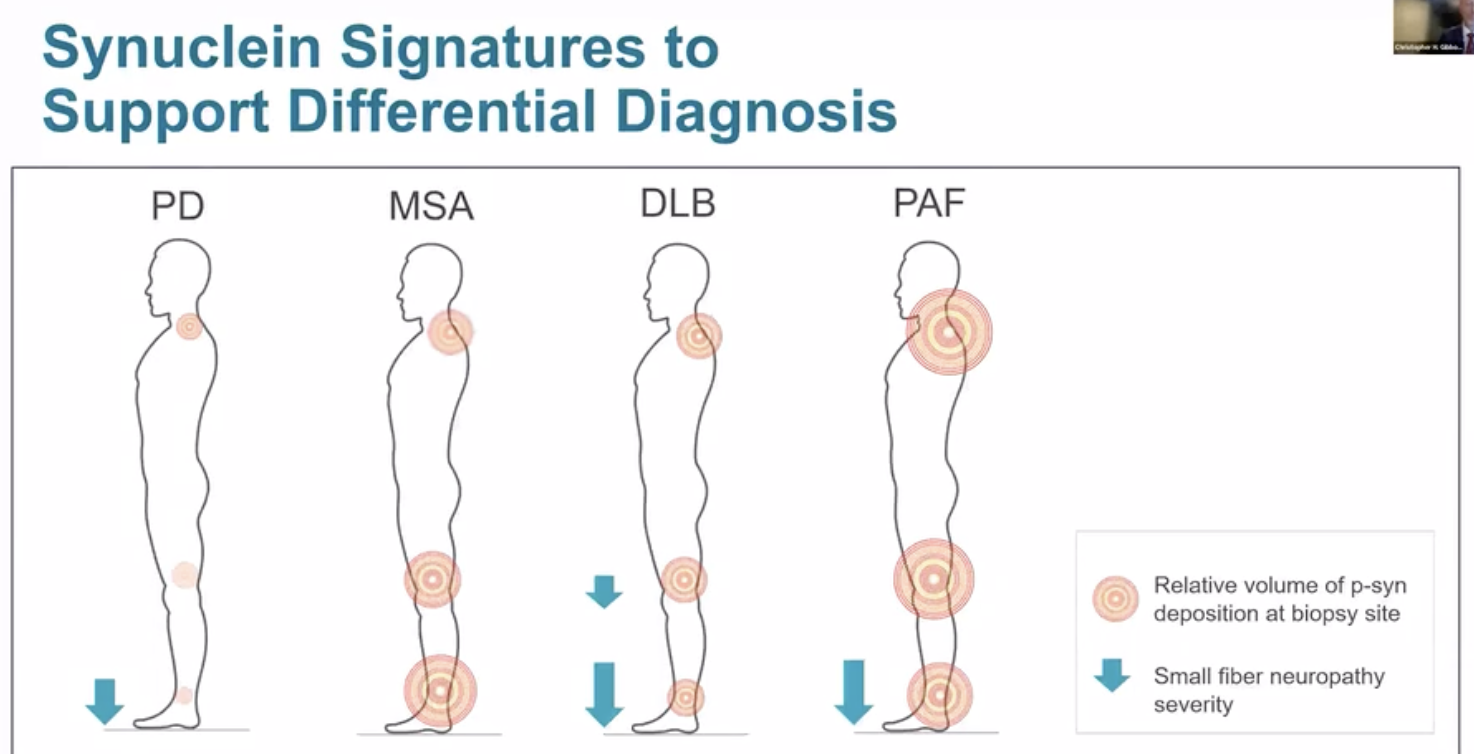
Preliminary data from the Gibbons NIH study revealed information, not just on synuclein deposition, but also on how nerve fiber density may possibly be used in the future to further differentiate the syndromes.
It is possible that the data from the skin biopsies will prove important beyond just the presence or absence of phosphorylated synuclein. The volume of synuclein discovered in each body region may provide critical clues for diagnosis. For example, in Parkinson’s disease, the neck region (preliminarily) seems to yield the largest amount synuclein compared to the other biopsy sites. Additionally, nerve loss, also referred to as small fiber neuropathy, may have different patterns of occurrence based on the (specific) underlying parkinsonian disorder. This additional information is summarized in the illustration above which proved to be an interesting discussion point during the AAN annual meeting.
What is a DaTScan?
The picture above is a nice teaching image of a DaT scan taken from Cedars Sinai, California.
In a January 14, 2011 press release ‘GE Healthcare announced the US Food and Drug Administration (FDA) approved DaTscan™ (Ioflupane I 123 Injection), as a radiopharmaceutical agent intended for use with single photon emission computed tomography (SPECT), specifically for the detection of dopamine transporters (DaT) in the brains of adult patients with suspected Parkinsonian syndromes. ‘ It was NOT approved as a test to diagnose Parkinson’s disease. The radiotracer is visualized by a special detector referred to as a ‘gamma camera.’
The trick with a DaT scan is that it "tags" a spot in the brain’s cells where dopamine attaches. So what does DaT really show? DaT shows the density of healthy dopamine neurons (brain cells).
If your DaTscan lights up robustly on both sides of the brain, this is considered a normal pattern. If your DaT lights up differently (asymmetrically) on each side of the brain, this may be a clue you have some ‘other’ form of parkinsonism.
In some cases the DAT scan may be ‘faded’ on both sides and this may be a clue to the diagnosis of one of the parkinsonian syndromes although much less is known about this pattern than other scan findings.
The DaTscan uses an Ioflupane I 123 injection (also known as the drug phenyltropane) as a radiopharmaceutical agent. A technician injects the compound into a person’s vein. The radiopharmaceutical then travels to the brain and marks the dopamine transporter. A camera is used to take a picture which is what is shown above.
The DaTscan scan provides important information on the ‘function of the brain.’ This is different than a MRI which gives information on the structure of the brain— what it looks like.
What is the most recent FDA labeling for DaT?
“DAT scanning is considered an “adjunct to other diagnostic evaluations for striatal dopamine transporter visualization using single photon emission computed tomography (SPECT) brain imaging in adult patients with:
- suspected Parkinsonian syndromes (PS) or
- suspected dementia with Lewy bodies (DLB)”
“A normal image is inconsistent with clinical conditions associated with PS and an abnormal image is consistent with clinical conditions associated with PS.”
Should I get a skin biopsy or DaTscan for diagnosis of Parkinson’s disease?
This picture from a BBC article from ‘Science Focus.’
One of the most frequently asked questions about Parkinson’s disease is whether or not to pursue a DaTscan or skin biopsy to ‘confirm a diagnosis of Parkinson’s disease.’
In most cases DaTscan and also the skin test are NOT necessary for diagnosis— also remember that a skin tests and DaTscans will NOT differentiate between the many PD syndromes.
Here are some tips which may be helpful if you are struggling with the decision of whether to pursue a DaT scan or a skin biopsy:
If you respond to dopaminergic therapy (levodopa etc.) in most cases a DaT scan or skin biopsy is not necessary,
If you already have a diagnosis of Parkinson’s disease and after many years of disease you begin to have symptoms that are no longer responsive to dopamine, you do NOT need a skin biopsy or DaTscan.
If your team is considering deep brain stimulation (DBS) implant for you and cannot differentiate essential tremor from Parkinson’s disease; and if understanding the diagnosis will impact their choice of brain targets for a future surgery—then a skin biopsy or DaTscan may be helpful.
If your clinical team is struggling to tell the difference between essential tremor or parkinsonism, skin biopsy or DaT scanning may be helpful.
If you have bipolar disorder or have been exposed to a dopamine blocking drug, a DaTscan may help you and your clinical team to determine if there is a drug induced parkinsonism. In cases of drug induced parkinsonism, usually the skin biopsy or DaTscan are ‘normal.’ Something worthy of consideration— Though rare, it is possible to have both Parkinson’s disease and drug induced parkinsonism (at the same time) and this is why a ‘clinical examination and expert opinion’ may be a useful adjunct if there is complexity to your symptoms or presentation.
It would be great if there were more studies and data available which directly compared skin biopsy to DaT, as this information could be useful in ‘shared decision making.’ At the AAN 2023 there was a small study presented in abstract form by Urval et. al. ‘DaTscan results was congruent with clinician’s suspected diagnosis in 15/24 cases (62.5%). Skin biopsy results were congruent with a clinician’s suspected diagnosis in 20/24 cases (83.3%). In two cases, the skin biopsy was positive for p-syn deposition where a synucleinopathy was not suspected. In 8 patients where the DaTscan was negative but clinician suspicion for a synucleinopathy was high, skin biopsies were positive.’ This study was too small in size to use in guiding decision making in clinical practice, however as more data from multi-country groups are published, this will enlighten ‘bedside decision making.’
Why is a DaTscan commonly misread or misinterpreted?
This YARN Futurama 1999 picture reminds us that since DaT is interpreted by the ‘eyes’ of the reader it can be prone to error. It should also not be interpreted without the relevant clinical examination and history.
In many cases the DaTscan is misinterpreted because the result is dependent on the clinician or radiologist’s eye— in other words, ‘there may be no hard numbers to assist in the interpretation.
It is critical when interpreting a DaT study, to take into consideration the history, clinical symptoms and what side of the body the symptoms are most bothersome (or pronounced); usually there is less DaT uptake on the side of the brain opposite the side of the body that is most affected. More recently, and especially for use in research, numeric values may be provided by advanced software packages which may help a clinician interpret the ‘asymmetry’ of the findings. These types of software packages may not be available at all locations where DaT is performed and interpreted.
Finally, DaT scanning now has a ‘generic’ product (like a generic drug) which may in some locales prove to be more cost effective.
What is the most recent data on DaTscan and Parkinson’s?
This figure by Schwarz in Journal of Nuclear Medicine in 2004 reminds us that data on DaT and DaT binding has been around a long time.
A recent comment by Dr. Bajal appeared in the journal Neurology which detailed challenges and opportunities when using DaT scanning.
DaT had good diagnostic accuracy in Parkinson’s disease.
Clinical trial error rates with diagnosis of Parkinson’s clinically may be as high as 15% and DaT helps to resolve these issues.
A blinded analysis of DAT had ‘high false positive and negative error rates in PD diagnosis and poor concordance between ‘movement disorder experts.’ This finding may have been underpinned by challenges presented by the concordance between rating neurologists. Additionally, SWEDD cases may be tricky to identify on clinical grounds alone.
The indication for DaTScan is NOT for a diagnosis of PD. DaT just tells you if there is an issue with the dopaminergic transporter and the likelihood there is some form of parkinsonism.
The prescribing information for DaT: ‘Visualization of the dopamine transporter (DaT) distribution within the striatum.’
Diagnosis should be made by clinicians and NOT by a scan.
There is a 1 in 7500 possible cancer risk with DaT, however this number likely could use more data to confirm.
*Notes on the 1 in 7500 risk in the literature: In the label DaT effective dose is ~4-6 mSv for a 3-5 mCi dose (including attenuation CT) which compares to 3-4 mSv per year average natural radiation exposure.
DaTscan is roughly equivalent to 1 year of natural radiation; Nguyen 2011 et al.
Should I get a Syn-One Skin Biopsy or a DaTscan?
Which one should I choose? Or do I really need one of these tests?
Here are some questions to ask yourself and to ask your doctor (or clinical team) to help guide ‘shared decision making.’ Spoiler alert: There is not a one size fits all when choosing a test and perhaps the most important step is deciding whether you actually need a test.
First, there are no direct comparative tests of DaT vs. a skin biopsy so the most important step is to have a detailed discussion with your doctor or clinician (only one very small study so far available in abstract form presented at the AAN Boston 2023 meeting).
Am I responding appropriately to levodopa therapy? A Syn-One or DaTscan is usually not necessary for diagnosis— if you have a good response to medications.
Have I been examined by a neurologist, and do I meet the criteria for Parkinson’s disease diagnosis? A Syn-One or DaTscan is usually not necessary for diagnosis. These tests may in some cases help clarify a diagnosis.
Do I have bipolar disorder and/or have I been exposed to a dopamine blocking drug? A Syn-One or DaTscan may help clarify a diagnosis of drug induced parkinsonism.
Do I have ‘intentional or essential tremor’ with my Parkinson’s symptoms? A Syn-One or DaTscan may help clarify the diagnosis. Additionally, this may be important for choosing the optimal brain target if you are considering deep brain stimulation (DBS) or focused ultrasound therapy (FUS).
Have you had Parkinson’s disease, but observed that you have not been ‘progressing.’ Non-progressive Parkinson’s disease is unusual and may raise ‘red flags’ there may be an incorrect diagnosis. There are conditions which can mimic Parkinson’s disease and a Syn-One skin test or DaTscan may help to clarify the diagnosis. In cases where the DaT is normal, folks have referred to this condition as a SWEDD (scan without evidence of dopamine deficiency). I am personally not a big fan of this term.
Finally, the Syn-One skin test or DaTscan may be important for ‘identification of eligibility’ for clinical trials. Additionally, these test may play a role in Parkinson’s disease drug development and may be important for the creation of Parkinson’s classification systems. Alzheimer’s disease is ~5 years ahead of Parkinson’s disease in developing classification systems and in Alzheimer’s they utilize a combination of MRI scanning, amyloid scanning and a blood test called p-tau. Close your eyes and imagine— in a few years we will ‘likely’ be following a similar classification and/or staging procedure for Parkinson’s disease.
Michael Okun is the author of this post and co-editor of the parkinsonsecrets.com blog. He has written 14 books and most recently Ending Parkinson’s Disease, Living with Parkinson’s Disease and Parkinson’s Disease Treatment: 10 Secrets to a Happier Life.
Jonny Acheson is the website artist, a physician and a person with Parkinson’s disease.
Selected References:
Gibbons C, Wang N, Rajan S, Kern D, Palma JA, Kaufmann H, Freeman R. Cutaneous α-Synuclein Signatures in Patients With Multiple System Atrophy and Parkinson Disease. Neurology. 2023 Apr 11;100(15):e1529-e1539. doi: 10.1212/WNL.0000000000206772. Epub 2023 Jan 19. PMID: 36657992; PMCID: PMC10103107.
Liguori R, Donadio V, Wang Z, Incensi A, Rizzo G, Antelmi E, Biscarini F, Pizza F, Zou W, Plazzi G. A comparative blind study between skin biopsy and seed amplification assay to disclose pathological α-synuclein in RBD. NPJ Parkinsons Dis. 2023 Mar 4;9(1):34. doi: 10.1038/s41531-023-00473-5. PMID: 36871045; PMCID: PMC9985591.
Kuzkina A, Rößle J, Seger A, Panzer C, Kohl A, Maltese V, Musacchio T, Blaschke SJ, Tamgüney G, Kaulitz S, Rak K, Scherzad A, Zimmermann PH, Klussmann JP, Hackenberg S, Volkmann J, Sommer C, Sommerauer M, Doppler K. Combining skin and olfactory α-synuclein seed amplification assays (SAA)-towards biomarker-driven phenotyping in synucleinopathies. NPJ Parkinsons Dis. 2023 May 29;9(1):79. doi: 10.1038/s41531-023-00519-8. PMID: 37248217; PMCID: PMC10226020.
Siepmann T, Arndt M, Sedghi A, Szatmári S Jr, Horváth T, Takáts A, Bereczki D, Moskopp ML, Buchmann S, Skowronek C, Zago W, Woranush W, Lapusca R, Weidemann ML, Gibbons CH, Freeman R, Reichmann H, Puetz V, Barlinn K, Pintér A, Illigens BM. Two-Year observational study of autonomic skin function in patients with Parkinson's disease compared to healthy individuals. Eur J Neurol. 2023 May;30(5):1281-1292. doi: 10.1111/ene.15733. Epub 2023 Feb 24. PMID: 36773001.
Parnetti L, Bellomo G. On the Track of α-Synuclein in the Body: Skin Biopsies for Diagnosing Synucleinopathies? Neurology. 2023 Apr 11;100(15):691-692. doi: 10.1212/WNL.0000000000206881. Epub 2023 Jan 19. PMID: 36657990.
Donadio V, Incensi A, Rizzo G, Westermark GT, Devigili G, De Micco R, Tessitore A, Nyholm D, Parisini S, Nyman D, Tedeschi G, Eleopra R, Ingelsson M, Liguori R. Phosphorylated α-synuclein in skin Schwann cells: a new biomarker for multiple system atrophy. Brain. 2023 Mar 1;146(3):1065-1074. doi: 10.1093/brain/awac124. PMID: 35552610.
Kim JY, Illigens BM, McCormick MP, Wang N, Gibbons CH. Alpha-Synuclein in Skin Nerve Fibers as a Biomarker for Alpha-Synucleinopathies. J Clin Neurol. 2019 Apr;15(2):135-142. doi: 10.3988/jcn.2019.15.2.135. PMID: 30938106; PMCID: PMC6444158.
Gibbons CH, Freeman R, Bellaire B, Adler CH, Moore D, Levine T. Synuclein-One study: skin biopsy detection of phosphorylated α-synuclein for diagnosis of synucleinopathies. Biomark Med. 2022 May;16(7):499-509. doi: 10.2217/bmm-2021-0646. Epub 2022 Mar 11. PMID: 35272481; PMCID: PMC9169016.
Wang N, Gibbons CH, Lafo J, Freeman R. α-Synuclein in cutaneous autonomic nerves. Neurology. 2013 Oct 29;81(18):1604-10. doi: 10.1212/WNL.0b013e3182a9f449. Epub 2013 Oct 2. PMID: 24089386; PMCID: PMC3806913.
Avena R, Picillo M, Pellecchia MT, Barone P, Erro R. Off-label DaT-SCAN use: a single-center, real-world study. Neurol Sci. 2023 Jul;44(7):2579-2580. doi: 10.1007/s10072-023-06730-y. Epub 2023 Mar 9. PMID: 36892664.
Constantinides VC, Souvatzoglou M, Paraskevas GP, Chalioti M, Stefanis L, Kapaki E. Dopamine transporter SPECT imaging in Parkinson's disease and atypical Parkinsonism: a study of 137 patients. Neurol Sci. 2023 May;44(5):1613-1623. doi: 10.1007/s10072-023-06628-9. Epub 2023 Jan 20. PMID: 36658411.
Nabizadeh F, Sodeifian F, Pirahesh K. Olfactory dysfunction and striatal dopamine transporter binding in motor subtypes of Parkinson's disease. Neurol Sci. 2022 Aug;43(8):4745-4752. doi: 10.1007/s10072-022-06110-y. Epub 2022 May 4. PMID: 35508569.
Bega D, Kuo PH, Chalkidou A, Grzeda MT, Macmillan T, Brand C, Sheikh ZH, Antonini A. Clinical utility of DaTscan in patients with suspected Parkinsonian syndrome: a systematic review and meta-analysis. NPJ Parkinsons Dis. 2021 May 24;7(1):43. doi: 10.1038/s41531-021-00185-8. PMID: 34031400; PMCID: PMC8144619.
Isaacson JR, Brillman S, Chhabria N, Isaacson SH. Impact of DaTscan Imaging on Clinical Decision Making in Clinically Uncertain Parkinson's Disease. J Parkinsons Dis. 2021;11(2):885-889. doi: 10.3233/JPD-202506. PMID: 33554925; PMCID: PMC8150650.
Śmiłowska K, Burzyńska-Makuch M, Brockhuis B, Piekarski R, Friedman A, Popek A, Sławek J. Neuroimaging in Parkinson's Disease: necessity or exaggeration? Neurol Neurochir Pol. 2021;55(6):536-548. doi: 10.5603/PJNNS.a2021.0068. Epub 2021 Oct 12. PMID: 34637136.